Uncovering, demonstrating, and communicating value
Research studies with a difference.
Real-world data have the power to inform better business decisions that can transform patients' lives, but only when expertly reviewed and leveraged to provide robust and insightful evidence. With an industry-leading multidisciplinary team, direct access to rich data sets, and superior analytics capabilities, we are well equipped to support and deliver projects that meet your objectives.

Our team is our difference.
Unlike other providers, we employ our teams to provide scientific rigor across the entire strategic process, applying insight and recommendations across ideation, design, implementation, and communication. This ensures that we produce reliable and compelling evidence that satisfies your stakeholders across the healthcare ecosystem, including regulators, payers, providers, and patients.
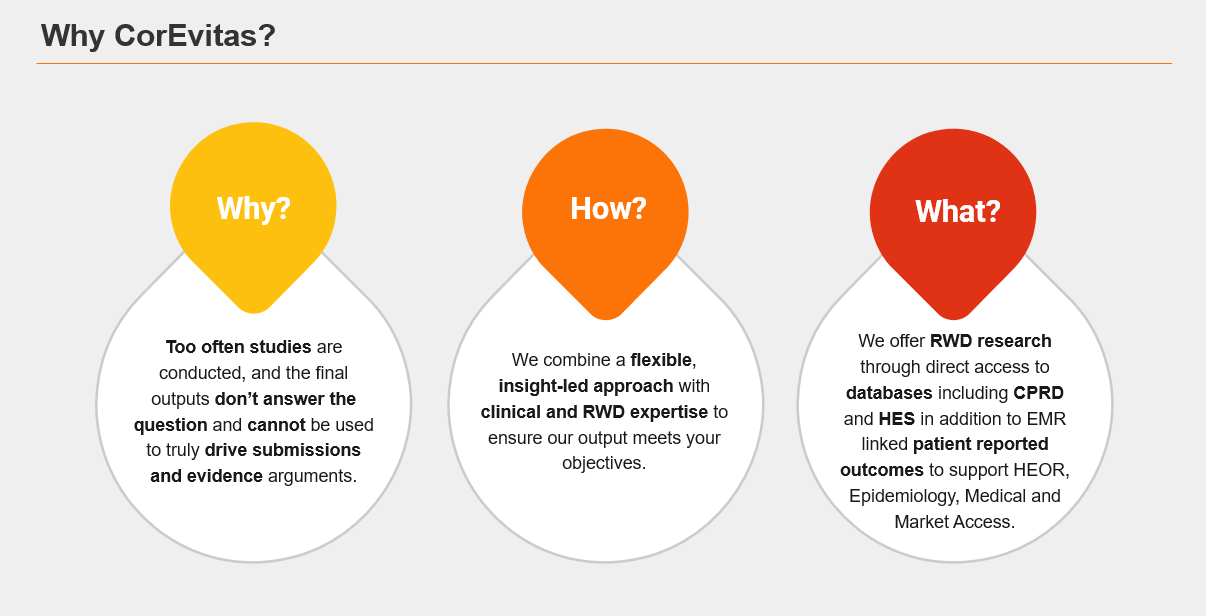
Our real-world evidence (RWE) solutions start with consultancy to help our clients to identify potential data gaps and the most optimal solutions for RWE generation. From determining the best research methodology to defining the most useful endpoints, our team provides an agile approach while applying the highest standards of scientific and academic practice.
The result? A fit-for-purpose study that asks and answers your most impactful scientific and commercial questions.
Our team boasts cross-functional expertise, bringing together expansive in-house experience in:
- Retrospective analysis of real-world data (RWD)
- Health economics and outcomes research
- Market access
- Medical affairs
- Epidemiology
- Wide-ranging clinical and therapeutic expertise
RWE across the product lifecycle
We can support your research needs at each stage of product development, from understanding the current landscape and areas of unmet need to maintaining access and demonstrating continued value.

A vast network of real-world data
We identify, access, and analyze real-world data from a multitude of diverse sources to answer your questions about disease epidemiology, burden, treatment, comparative effectiveness, and cost.
- Clinical Practice Research Datalink (CPRD), for which we hold a multi-study license
- Hospital Episode Statistics (HES), held in-house
- Cancer registry and systemic anti-cancer therapy (SACT)
Excellence in Evidence: CorEvitas in Numbers
We provide access to a international multidisciplinary team well-versed in major therapeutic areas, enabling us to deliver local solutions with global insight.

A breadth of expertise

Experience and capabilities in peer-reviewed research
Dr Jennifer Davidson
Head of Research
Jennifer is Head of Research and provides epidemiological expertise for our study design and execution. She holds a Masters in Public Health Research, is currently completing her PhD in Epidemiology, and previously worked at Public Health England leading a team of epidemiologists, analysts, and data managers. Jennifer has extensive experience in peer review publication, with articles published in various journals.
Dr. Caoimhe Rice
Principal Physician Economist
Caoimhe is the Principal Physician Economist in RWE within the CorEvitas Specialty EMR Data Team. She offers a unique perspective as a physician and health economist, with degrees in medicine and history and philosophy of science from the University of Cambridge and a masters’ in health economics from the University of York. She has over 10 years’ experience practicing hospital medicine, alongside a wealth of experience in health technology assessments both within randomized controlled trials and using large real-world clinical datasets in observational research. Caoimhe has led rare disease studies including in sickle cell disease and systemic mastocytosis. She is passionate about equity in health and care and applied this to her work at CorEvitas, investigating inequity in valve procedures and cancer treatment.
Dr. Simon Wan Yau Ming
Director, Strategic Solutions
Dr. Simon Wan Yau Ming provides strategic consultation regarding evidence planning and generation to address stakeholder needs for market access. Additionally, he participates in the design, analysis, and interpretation of studies with a specific focus on research questions requiring real-world evidence. Dr. Wan Yau Ming previously worked as a general practitioner and accident and emergency clinician, and has published in journals including Respirology, Journal of Thoracic Disease, and Pulmonary Therapy.
Dr. Sara Carvalho
Biostatistician
Dr. Sara Carvalho is a biostatistician at CorEvitas and holds a first-class degree in Biomedical Engineering. She has broad experience in the academic environment; after concluding a PhD in Biostatistics at the University of Maastricht, she extended her skills within the genomics field at the Cancer Genetic Epidemiology Institute at the University of Cambridge. Her recent transition into industry has been rewarding, as her knowledge and expertise have been used to derive meaningful information from real-world evidence data, providing statistical expertise into client research project design and execution.
Mico Hamlyn
Lead Data Analyst
Mico is the team’s Lead Data Analyst. He holds a Bachelors degree in Natural Sciences from the University of Cambridge and a Masters degree in Public Health from Imperial College London. Mico brings his multi-sector expertise to our team and projects, having worked for the NHS in modeling workforce demand and supply, in academia working on temporospatial epidemiology, and in industry working with real-world evidence.





